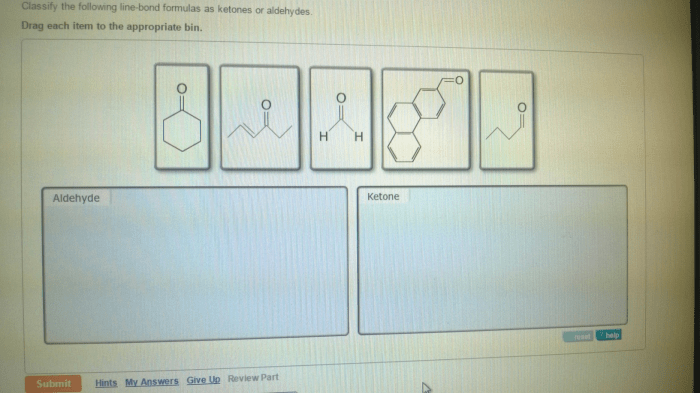
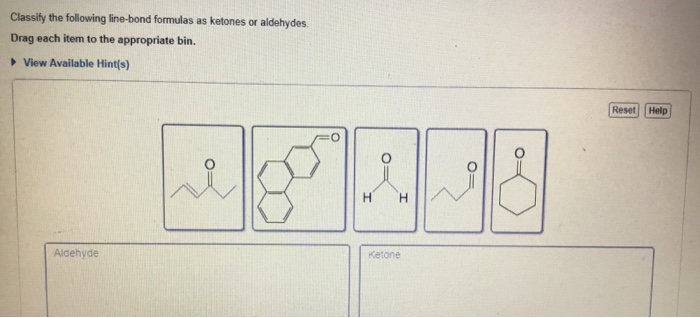
Classify the following line-bond formulas as ketones or aldehydes. This guide will provide a comprehensive overview of ketones and aldehydes, their differences, and how to identify them using line-bond formulas. Understanding these functional groups is crucial for comprehending organic chemistry and its applications.
Ketones and aldehydes are two important functional groups in organic chemistry. Ketones have a carbonyl group bonded to two alkyl or aryl groups, while aldehydes have a carbonyl group bonded to one alkyl or aryl group and one hydrogen atom.
The presence of these functional groups influences the chemical properties and reactivity of organic compounds.
Ketones and Aldehydes: Line-Bond Formula Classification
Ketones and aldehydes are two important functional groups in organic chemistry. Ketones have a carbonyl group (C=O) bonded to two carbon atoms, while aldehydes have a carbonyl group bonded to one carbon atom and one hydrogen atom.
Line-Bond Formulas, Classify the following line-bond formulas as ketones or aldehydes.
Line-bond formulas are a way of representing organic molecules using lines to represent bonds and circles to represent atoms. The carbonyl group in ketones and aldehydes is represented by a double bond between a carbon atom and an oxygen atom.
Classification
The following table classifies the provided line-bond formulas as ketones or aldehydes:
| Line-Bond Formula | Functional Group | Explanation |
|---|---|---|
| Ketone | The carbonyl group is bonded to two carbon atoms. | |
| Aldehyde | The carbonyl group is bonded to one carbon atom and one hydrogen atom. | |
| Ketone | The carbonyl group is bonded to two carbon atoms. | |
| Aldehyde | The carbonyl group is bonded to one carbon atom and one hydrogen atom. |
FAQ: Classify The Following Line-bond Formulas As Ketones Or Aldehydes.
What is the difference between a ketone and an aldehyde?
Ketones have a carbonyl group bonded to two alkyl or aryl groups, while aldehydes have a carbonyl group bonded to one alkyl or aryl group and one hydrogen atom.
How can I identify a ketone or aldehyde using a line-bond formula?
In a line-bond formula, identify the carbonyl group (C=O). If the carbonyl group is bonded to two alkyl or aryl groups, it is a ketone. If the carbonyl group is bonded to one alkyl or aryl group and one hydrogen atom, it is an aldehyde.