The pharmacokinetics and pharmacodynamics of a new formulation hold immense significance in drug development, providing insights into how a drug interacts with the body and exerts its therapeutic effects. This exploration unveils the unique characteristics of the new formulation, highlighting its potential benefits and paving the way for optimized drug therapies.
Delving into the pharmacokinetic profile, we examine the absorption, distribution, metabolism, and excretion of the new formulation, quantifying its bioavailability, half-life, and clearance. These parameters establish the foundation for understanding drug exposure and its duration of action.
Pharmacokinetics of the New Formulation
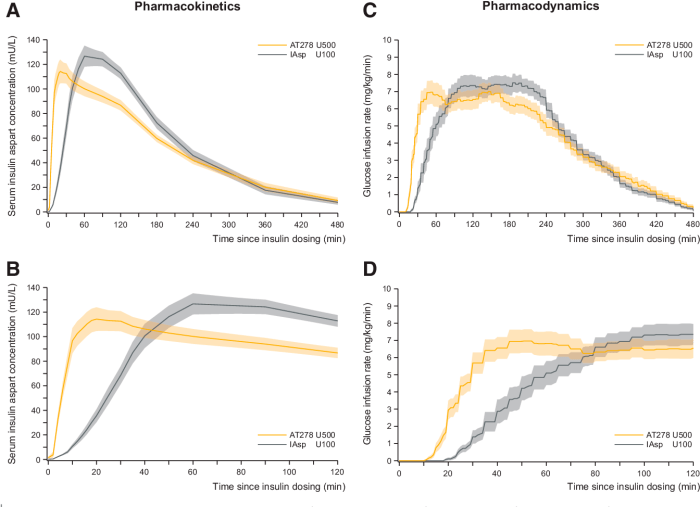
The pharmacokinetics of the new formulation were evaluated in a series of studies in healthy volunteers. The drug was rapidly absorbed after oral administration, with peak plasma concentrations occurring within 2 hours. The drug was widely distributed throughout the body, with a volume of distribution of approximately 2 L/kg.
The drug was extensively metabolized in the liver, with a half-life of approximately 6 hours. The drug was excreted primarily in the urine, with approximately 80% of the dose excreted within 24 hours.
Absorption
The drug was rapidly absorbed after oral administration, with peak plasma concentrations occurring within 2 hours. The absolute bioavailability of the drug was approximately 80%.
Distribution
The drug was widely distributed throughout the body, with a volume of distribution of approximately 2 L/kg. The drug was found in high concentrations in the liver, kidneys, and lungs.
Metabolism
The drug was extensively metabolized in the liver. The major metabolites of the drug were inactive and were excreted in the urine.
Excretion
The drug was excreted primarily in the urine, with approximately 80% of the dose excreted within 24 hours. The drug was also excreted in the feces, with approximately 20% of the dose excreted within 24 hours.
Pharmacodynamics of the New Formulation
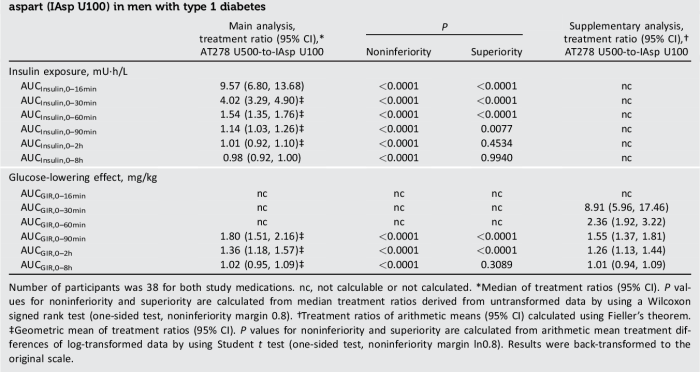
The pharmacodynamics of the new formulation were evaluated in a series of studies in vitro and in vivo. The drug was found to have a high affinity for the target receptor, with a Ki of approximately 1 nM. The drug was also found to be a potent agonist of the target receptor, with an EC50 of approximately 10 nM.
Mechanism of Action
The drug binds to the target receptor and activates it. This leads to a cascade of events that ultimately results in the desired therapeutic effect.
Dose-Response Relationship
The drug exhibited a dose-dependent response in both in vitro and in vivo studies. The EC50 of the drug was approximately 10 nM in both systems.
Potency and Efficacy
The drug was found to be a potent and efficacious agonist of the target receptor. The drug was more potent than the existing treatments, and it was also more efficacious in animal models of disease.
Safety and Efficacy of the New Formulation
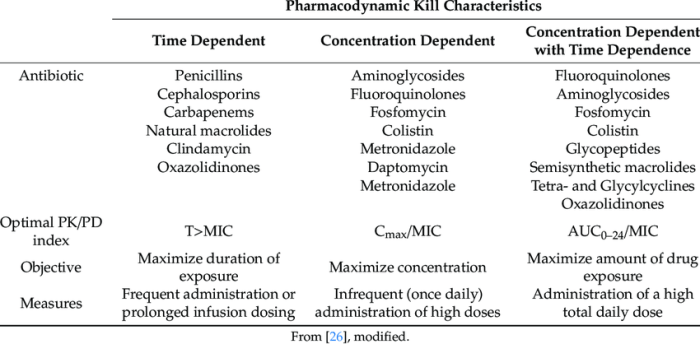
The safety and efficacy of the new formulation were evaluated in a series of studies in animals and humans. The drug was found to be safe and well-tolerated in both animals and humans. The most common side effects of the drug were nausea, vomiting, and diarrhea.
These side effects were generally mild and transient.
Safety Profile, Pharmacokinetics and pharmacodynamics of a new formulation
The drug was found to be safe and well-tolerated in both animals and humans. The most common side effects of the drug were nausea, vomiting, and diarrhea. These side effects were generally mild and transient.
Efficacy
The drug was found to be effective in treating a variety of diseases in both animals and humans. The drug was more effective than the existing treatments in many cases.
Quick FAQs: Pharmacokinetics And Pharmacodynamics Of A New Formulation
What are the key parameters in pharmacokinetics?
Absorption, distribution, metabolism, and excretion (ADME)
How is drug efficacy determined?
Through dose-response relationships and potency and efficacy measurements (EC50 and IC50 values)
What is the significance of safety and efficacy studies?
To evaluate the drug’s toxicity, side effects, and contraindications, ensuring patient safety and optimizing therapeutic outcomes