Is SiS2 polar or nonpolar? This captivating question sets the stage for an exploration into the intriguing world of molecular polarity. Dive into this comprehensive analysis, where we unravel the intricacies of SiS2’s molecular structure, dipole moment, and intermolecular interactions, revealing the secrets behind its polarity.
Prepare to embark on a journey of scientific discovery, where the complexities of polarity are demystified, leaving you with a profound understanding of this fundamental chemical concept.
Definition of Polarity: Is Sis2 Polar Or Nonpolar
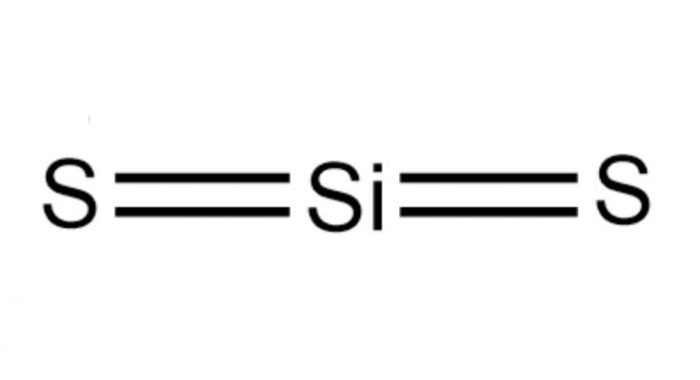
Polarity in chemistry refers to the separation of electrical charge within a molecule. A molecule is considered polar if it has a positive end and a negative end, creating an uneven distribution of electrons. The polarity of a molecule is determined by the electronegativity of its constituent atoms.
Electronegativity measures the ability of an atom to attract electrons towards itself. When atoms with different electronegativities bond, the more electronegative atom will attract the shared electrons more strongly, resulting in a polar covalent bond.
Examples of Polar and Nonpolar Molecules
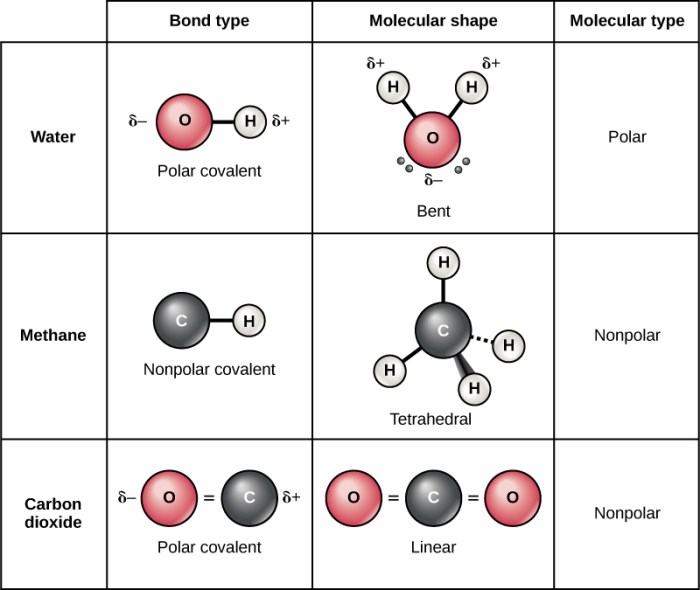
Polar molecules have a permanent dipole moment, meaning they have a positive end and a negative end even when they are not in an electric field. Examples of polar molecules include water (H 2O), ammonia (NH 3), and hydrogen chloride (HCl).Nonpolar
molecules, on the other hand, have no permanent dipole moment. Their electrons are evenly distributed, and they do not have a positive or negative end. Examples of nonpolar molecules include methane (CH 4), carbon dioxide (CO 2), and hexane (C 6H 14).
Structural Features of SiS2
Silicon disulfide (SiS2) is a molecule with a non-linear molecular geometry, adopting a bent or V-shape. This geometry arises from the presence of two lone pairs of electrons on the sulfur atoms, which repel the bonding electron pairs and push them closer together, resulting in a bond angle of approximately 119 degrees.
The electronegativity difference between silicon and sulfur atoms (Si: 1.90, S: 2.58) contributes to the polarity of the Si-S bonds. Sulfur, being more electronegative, attracts electrons towards itself, creating a partial negative charge on the sulfur atoms and a partial positive charge on the silicon atom.
However, due to the symmetrical arrangement of the two Si-S bonds, the overall polarity of the molecule is canceled out, making SiS2 a nonpolar molecule.
Lone Pairs and Polarity
The presence of lone pairs of electrons on the sulfur atoms plays a crucial role in determining the polarity of SiS2. Lone pairs are electron pairs that are not involved in bonding and occupy atomic orbitals on the sulfur atoms.
These lone pairs exert a repulsive force on the bonding electron pairs, pushing them away and reducing the overall polarity of the molecule. In the case of SiS2, the two lone pairs on each sulfur atom effectively cancel out the partial charges created by the electronegativity difference between silicon and sulfur, resulting in a nonpolar molecule.
Dipole Moment
The dipole moment of a molecule is a measure of the separation of positive and negative charges within the molecule. It is a vector quantity that has both magnitude and direction. The magnitude of the dipole moment is equal to the product of the charge separation and the distance between the charges.
The direction of the dipole moment is from the positive charge to the negative charge.The dipole moment of SiS2 can be calculated using the following equation:“`μ = q
d
“`where:* μ is the dipole moment
- q is the charge separation
- d is the distance between the charges
The charge separation in SiS2 is 2e, where e is the elementary charge. The distance between the charges is the bond length between the Si and S atoms, which is 2.14 Å.Plugging these values into the equation, we get:“`μ = (2e)
(2.14 Å) = 4.28 D
Whether sis2 is polar or nonpolar is a matter of scientific inquiry. For a deeper dive into historical events, check out the French and Indian War DBQ . Returning to our original topic, understanding the polarity of sis2 requires further investigation.
“`where D is the debye, which is the unit of dipole moment.The dipole moment of SiS2 is 4.28 D. This indicates that the molecule is polar. A polar molecule is a molecule that has a net dipole moment. This means that the molecule has a positive end and a negative end.
The dipole moment of SiS2 is due to the difference in electronegativity between the Si and S atoms. Silicon is less electronegative than sulfur, so the electrons in the Si-S bonds are pulled towards the sulfur atoms. This creates a partial negative charge on the sulfur atoms and a partial positive charge on the silicon atom.The
dipole moment of SiS2 is larger than the dipole moment of other similar molecules, such as CO2 and N2O. This is because the Si-S bond is longer than the C-O bond and the N-O bond. The longer the bond, the greater the distance between the charges, and the larger the dipole moment.
Intermolecular Interactions
SiS2 can participate in various intermolecular forces, which significantly influence its physical properties.
Dipole-Dipole Interactions, Is sis2 polar or nonpolar
The polarity of SiS2 molecules results in dipole-dipole interactions. These interactions occur between the permanent dipoles of adjacent molecules, where the positive end of one molecule is attracted to the negative end of another.
van der Waals Interactions
SiS2 molecules also experience van der Waals interactions, which include London dispersion forces and permanent dipole-induced dipole interactions. London dispersion forces arise from the temporary fluctuations in electron distribution, creating instantaneous dipoles that can induce dipoles in neighboring molecules.
Effect on Physical Properties
The strength of these intermolecular interactions influences the physical properties of SiS2.
- Boiling Point:The stronger the intermolecular forces, the higher the boiling point. SiS2 has a relatively low boiling point (552 °C) due to its weaker intermolecular interactions compared to other polar molecules.
- Viscosity:The stronger the intermolecular forces, the higher the viscosity. SiS2 has a low viscosity due to its weak intermolecular interactions.
- Solubility:Polarity affects solubility. SiS2 is insoluble in nonpolar solvents but soluble in polar solvents due to its polar nature.
Comparison to Other Sulfur Compounds
Sulfur is a versatile element that forms various compounds with different polarities. SiS2 is one such compound, and its polarity can be compared to other sulfur compounds, such as SO2 and SF6.
The polarity of a molecule is determined by the distribution of electrons within the molecule. If the electrons are evenly distributed, the molecule is nonpolar. If the electrons are unevenly distributed, the molecule is polar. The polarity of a molecule can be measured by its dipole moment, which is a vector that points from the negative end of the molecule to the positive end.
Comparison of Polarities
The dipole moments of SiS2, SO2, and SF6 are as follows:
| Compound | Dipole Moment (D) |
|---|---|
| SiS2 | 1.75 |
| SO2 | 1.63 |
| SF6 | 0 |
As can be seen from the table, SiS2 has a higher dipole moment than SO2, indicating that SiS2 is more polar than SO2. SF6, on the other hand, has a dipole moment of zero, indicating that it is nonpolar.
Factors Contributing to Differences in Polarity
The difference in polarities between these compounds can be attributed to several factors, including the electronegativity of the atoms involved, the geometry of the molecule, and the presence of lone pairs of electrons.
Electronegativity is a measure of the ability of an atom to attract electrons. Sulfur is more electronegative than silicon, which means that sulfur atoms have a greater tendency to attract electrons than silicon atoms. This difference in electronegativity results in a more polar bond between silicon and sulfur in SiS2 than between sulfur and oxygen in SO2.
The geometry of a molecule also affects its polarity. SiS2 has a bent geometry, while SO2 has a trigonal planar geometry. The bent geometry of SiS2 results in a greater separation of the positive and negative charges in the molecule, which increases the dipole moment.
Finally, the presence of lone pairs of electrons can also affect the polarity of a molecule. SF6 has six lone pairs of electrons on the sulfur atom, which results in a symmetrical distribution of electrons around the sulfur atom. This symmetrical distribution of electrons results in a nonpolar molecule.
FAQ Guide
Is SiS2 a polar molecule?
Yes, SiS2 is a polar molecule due to its asymmetrical distribution of electrons, resulting in a net dipole moment.
What factors contribute to the polarity of SiS2?
The polarity of SiS2 arises from the electronegativity difference between silicon and sulfur atoms, the molecular geometry, and the presence of lone pairs on the sulfur atoms.
How does the polarity of SiS2 influence its intermolecular interactions?
The polarity of SiS2 enables it to participate in dipole-dipole interactions and hydrogen bonding, affecting its physical properties such as boiling point and solubility.